Micro-Bio-Battery
Experiment
Our goal of this experiment was to confirm the transfer of calcium ions between two types of droplets and the verification of the movement of protons that occurs consequently.
Materials and Equipment
Liquid paraffin
squalene
water
SDS(sodium dodecyl sulfate)
octadecyltrietoxysilane
Span80(called sorbitan monooleate)
EDTA(Ethylene Diamine Tetraacetic Acid)
Ca(OH)2
HCl
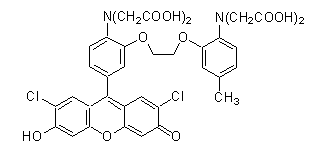
FCM(Fluoresbrite Carboxylate Microspheres)
Ionomycin
fluo3 and SPG pomping conector
*EDTA (Ethylenediaminetetraacetic acid)
By combining with divalent, trivalent and tetravalent metal ions, EDTA forms chelate complex stably. (Excerpted from Gifu Prefecture Board of Education and translated[1])
*FCM was bought from Polysciences.
*Fluo3, Ca2+ indicator, was bought from Dojindo Molecular Technologies, Inc.
The fluorescence characteristics of fluo3 are as follows. It is visible with excitation wavelength of 508nm and fluorescence wavelength of 527nm. When it bonds with Ca2+, its fluorescence intensity of fluo3 increases as much as 40 times. (Excerpted from Dojindo Molecular Technologies Inc. HP and translated[2])
*Ionomycin is antibiotics
It functions as ionophore and has antibacterial activity to gram-positive bacteria. It often bonds with divalent cations and utilized to compulsorily flow into cells (the ion selectivity is as follows: Ca2+>Mg2+>>Sr2+=Ba2+). It has high affinity towards Ca2+. It is also used in biological membrane transportation experiments of Ca2+. (Excerpted from Wako Pure Chemical Industries Ltd. HP and translated[3])
Procedure
1. Preparation of solutions
As continuous oil phase, a solution of liquid paraffin and squalene was used. The volume ratio of liquid paraffin to squalene was 1:1 and was prepared 5ml in total. Using high viscosity liquid paraffin stabilizes the droplets and mixing it with squalene that has relatively high viscosity makes the experiment proceed smoothly. Then 3.5mg (0.1 wt%) of SDS and 98.22mg (3wt%) of span80, which is a surfactant, were added. Because SDS is solid and it was difficult to dissolve it completely, the solution was warmed to 90 degrees Celsius for 30 minutes. The presence of SDS enhanced the dispensability of water droplets in oil (this solution was named solution A).
As water phase, two solutions in different conditions were prepared. For the first solution, 9mg of glucose was dissolved in pure water. In addition, EDTA and fluo3 were added and adjusted so that the concentration of each was 0.2 nM and 4.0μM (this was named solution B1). The reason why we used EDTA was clarifying that no calcium ions existed in solution B1. For the second solution, 27μl of 1.5M glucose solution mixed with 400μl of the 10wt% FMC (Fluoresbrite Carboxylate Microspheres) solution, which was washed with sodium hydroxide was prepared (this was named solution B2).
When preparing the water phase, FMS had to be calcified. Fluorescence beads in the FMC solution have carboxyl group and were provided as sodium compound. The compound was then immersed into dilute hydrochloric acid to separate carboxyl group and sodium. Consequently, it was reacted with calcium hydroxide neutralized with strong basic to generate fluorescence beads with calcified carboxyl group. The generated compound was then rinsed several times to wash off calcium ions that were not bonded with carboxyl group.
2. Saturate continuous oil phase with water
Solution A was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 (solution A:water=5.0ml:0.56ml) for 30 minutes. Solution A was saturated with water in advance in order to remove soluble impurities. (This avoids solution B1 and B2 from seeping into the continuous oil phase and affecting the fluorescence intensity observation at the end.) Then, the supernatant continuous oil phase was removed and distributed 1ml each into two micro-tubes, followed by centrifugation (5000rpm for 8min with a centrifugal separator). The supernatant solution was removed and used as continuous phase (the resulting solution was also named solution A).
3. Formation of water in oil emulsion(=W/O emulsion) by SPG pumping connector
Solutions A and B1 were each inserted into two syringes at the volume ratio of 4:1 (=600 μl:150μl in our experiment) and connected using the SPG pumping connector. The syringe of solution A was pushed towards solution B1 in order to coat the SPG porous surface of the SPQ pumping connector with solution A. In this way, solution B1 was forced through porous medium into solution A and solution B1 was dispersed. Through the process, the size of water droplets became almost the same as that of tunnels in the SPG pumping connector, which was about 2μm. Thus W/O emulsion was formed (named solution C1).
These figures are reffered from [4].
1. The continuous oil phase and the water phase were prepared and SPG pumping connecter was filled with the former in advance.
2. The syringe (piston) of the water phase was connected to the SPG pumping connecter.
3. The continuous oil phase was injected into the water phase.
4/5. The water phase was injected into the other side.
6. 1~5 was repeated until the size of the droplets was roughly 20 μl.
Reffered and translated from [4].
The same procedure was also conducted on solutions A and B2. (The resulting W/O emulsion was named solution C2). At this point, there should be two types of W/O emulsion (at the volume ratio of solution A:solution Bi = 4:1).
* i=1,2
4. Transfer of Calcium Ion using Ionomycin
Firstly, solutions C1 and C2 were mixed at the volume ratio of 1:1. Then two samples, one mixed with 0.1wt% ionomycin and the other with 0.02wt%, were prepared and extracted 100μl each (the solutions were named D1 and D2). Two additional samples made by mixing C1 & wt0.1 ionomycin and C1 & wt0.02% ionomycin were prepared as a contrast experiment (the solutions were named D3 and D4)
5. Observation
Solutions D1 ~ D4 were observed one hour and one day after preparation. Before observation, solution D was diluted 100 folds by solution A so that the final ratio of water phase:continuous oil phase was 1:400.
Preliminary Experiments
To conduct more efficient search and observation of W/O emulsion, we conducted some preliminary experiments before the main experiment.
These experiments were conducted with reference to the experiment by Sugiura and others (Sugiura et al. 2007).
Materials
Liquid paraffin
squalene
span80 (sorbitan monooleate)
SA (stearylamine)
calcein
Tris-HCl buffer
water
Experiments
1. Testing the practicality of the SPG pumping connector
The SPG (Shirasu Porous Glass) pumping connector is a device to make monodispersed W/O emulsion, in which droplets are uniform in size. Two kinds of SPG pumping connectors were prepared. The diameter of tunnels in one SPG pumping connector was 10μm and that of the other was 20μm. Although it is easier to observe bigger water droplets, it is more difficult for them to remain stable in general. To decide which SPG pumping connector to use in the main experiment, the stability of differently sized droplets was observed.
i. Preparation of solutions
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, Tris-HCl buffer (50mM,pH8) containing 0.4mM calcein was used.
ii. Saturate continuous oil phase with water
The continuous oil phase was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 for 30 minutes.
iii. Formation of W/O emulsion by SPG pumping connector
Continuous oil phase and water phase were each inserted into two syringes at the volume ratio of 10:1 and connected using the SPG pumping connector and were pumped until the size of the droplets became almost the same.
iv. Observation
Figure 1. Microscope photographs of droplets.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
From the results of the observation of W/O emulsion, the following conclusions were obtained.
・Droplets produced by the connector with 20μm tunnels were stable enough to exist.
・By procedure 3, the diameter of droplets became certainly uniform.
・With more pumping, the proportion of smaller droplets became high.
Considering these results, we decided to use the SPG pumping connector with 20μm tunnels and to pump 5 times in the main experiment.
From these pictures, it was found that the density of droplets was too high and that it was important to search for proper density of droplets for observation.
2. Searching for proper density of droplets for observation
As the ratio of water phase to continuous phase changes, the density of droplets changes and the difficulty of observation also changes. For more efficient observation, four conditions of dilution were tested.
In this experiment, materials and procedures were almost the same as preliminary experiment 1. Only the degree of dilution before observation was different. After pumping, W/O emulsion was diluted with continuous phase to the degree that the ratio of water phase to continuous phase was 30:1, 100:1 and 1000:1.
Figure 2.
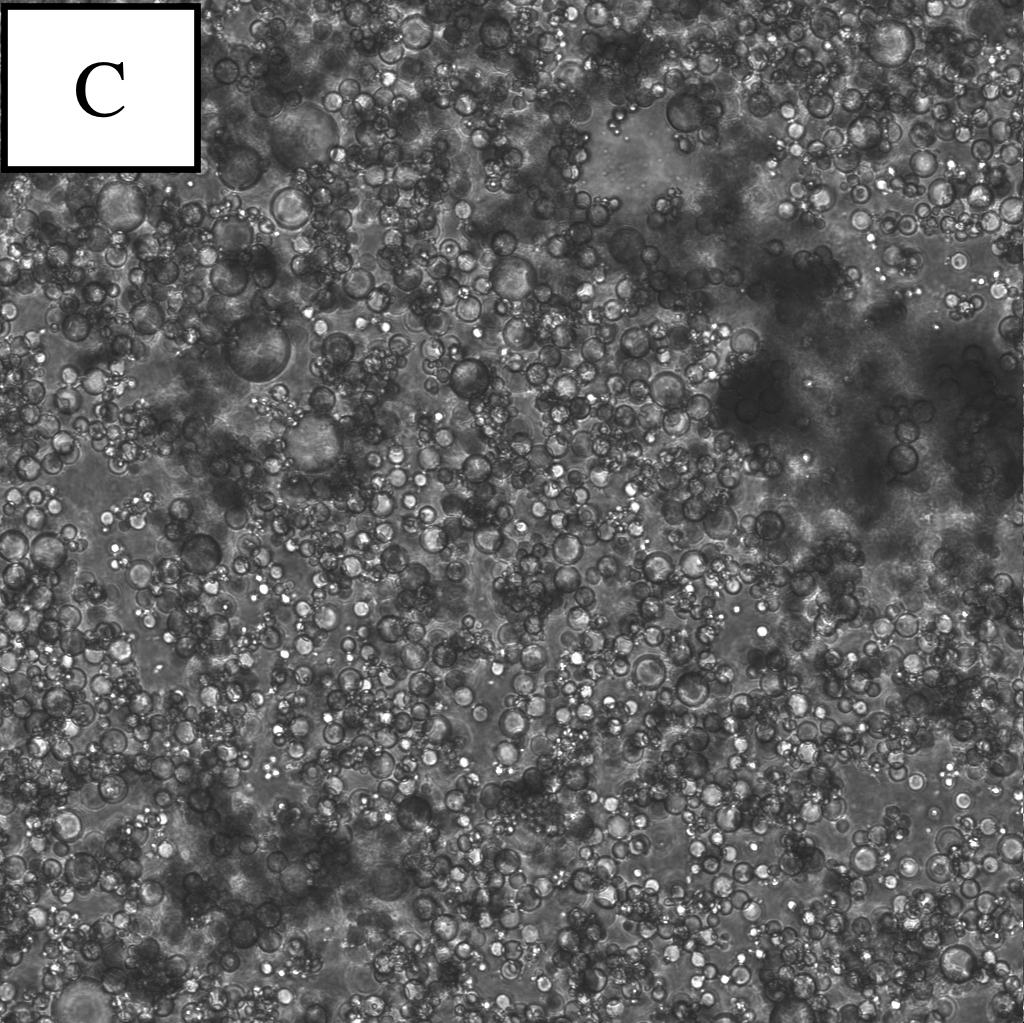
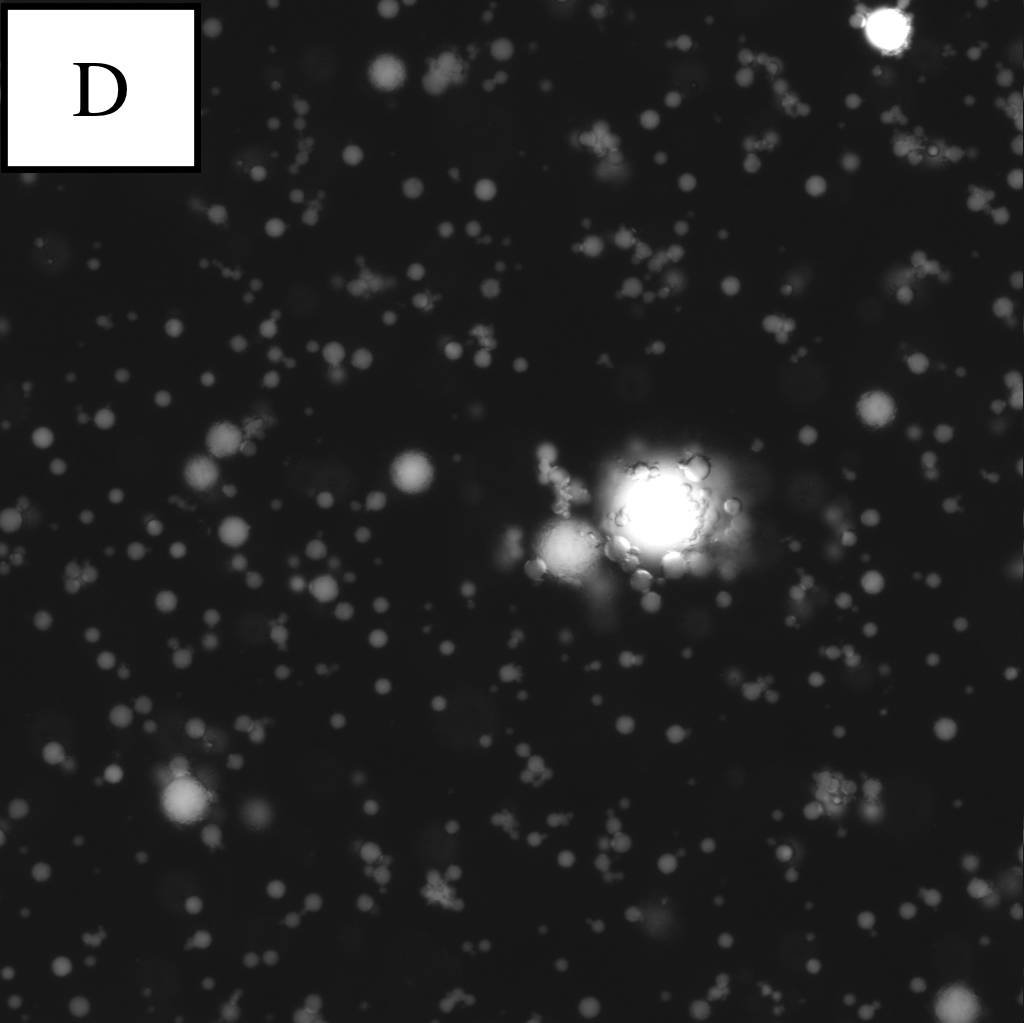
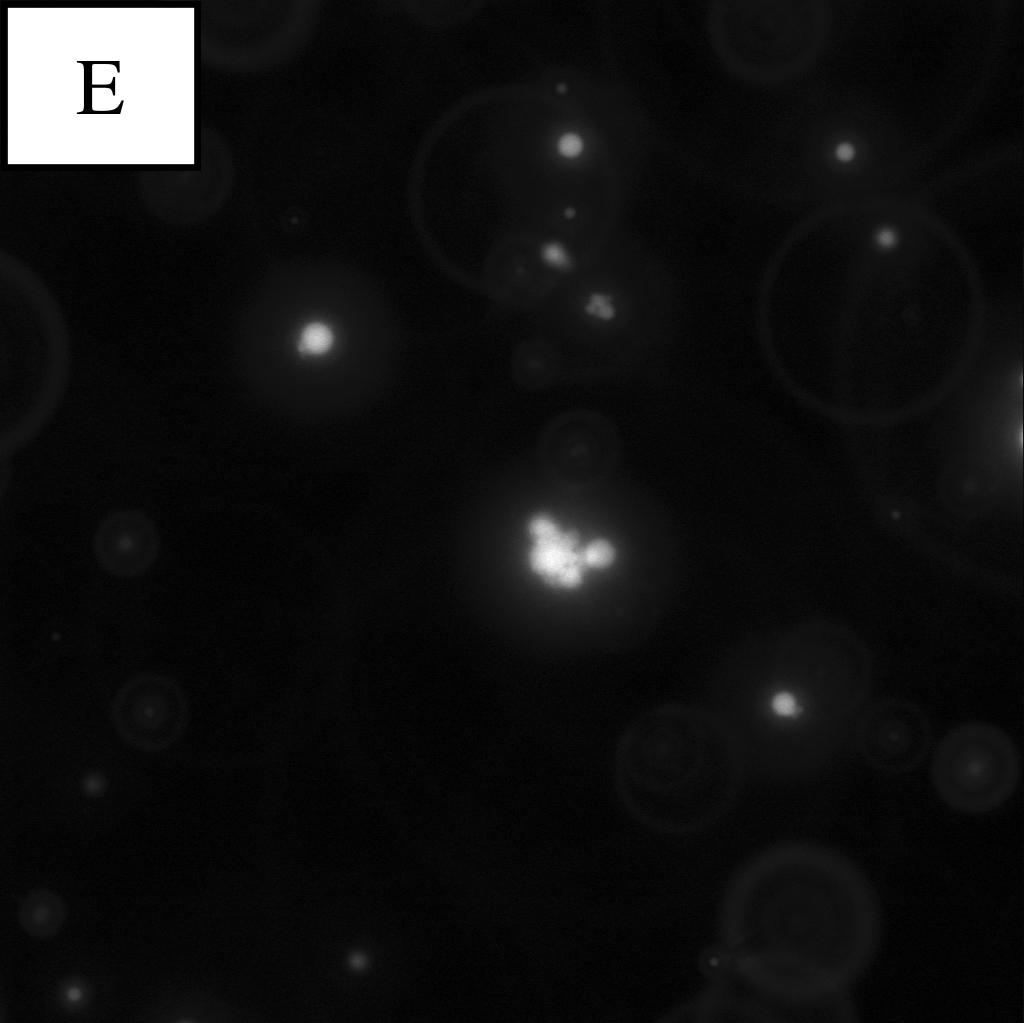
Microscope photographs of droplets at the different dilution rates.
Panel C is a picture taken in the bright field.
continuous oil phase:water phase=30:1
Panel D is a picture taken in the blue excitation light*.
continuous oil phase:water phase=100:1
Panel E is a picture taken in the blue excitation light*.
continuous oil phase:water phase=1000:1
*The wavelength of blue excitation light is close to the Maximal absorption wavelength of calcein. So in blue excitation light, calcein looks shining.
By observing W/O emulsion in these conditions, it was understood that the condition (water phase:continuous oil phase = 1:100~1000) was proper for observation.
3. Investigating the effect of Tris-HCl buffer to the stability of W/O emulsion
Tris-HCl buffer was used as water phase in the experiment by Sugiura. On the other hand, in our main experiment, Tris-HCl buffer was not proper because producing the difference of pH was important. To confirm whether W/O emulsion remains stable without buffer, and if not, to reconsider the conditions of the main experiment, we took place the following experiment.
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, the following solutions were used.
A) Tris-HCl buffer (pH9, 50mM)
B) Water with glucose (10mM) (No electrically charged particles exist. The concentration of particles in the solution was the same as that of 50mM Tris-HCl buffer)
C) Water
In solution A), stable W/O emulsion was observed. In other words, droplets repel each other and did not make clusters. In solutions B) and C), droplets were stuck together and many clusters were made. Thus, it became clear that in order for droplets to repel each other the existence of buffer was essential.
In this system, SA, which is a kind of amine, is the cause of repulsion between droplets and that of stability of W/O emulsion because amine is bound with a proton (H+ ion) ion in the solution and gets positive charge. It forms a part of the membrane of water droplets. Then, droplets get positive charge and repel each other.
From the above consideration, it became clear that in order for SA to work, providing SA with protons in the solution was essential. However, Tris-HCl buffer is not proper for the main experiment. Therefore, another kind of surfactant which causes repulsion between water droplets without ion interchange was needed.
Then, it occurred to us to use SDS instead of SA. SDS is surfactant made from strong acid and strong base, so SDS easily ionizes in water. Thus, it was expected to cause repulsion between water droplets in continuous oil phase.
When SDS was used as continuous oil phase in place of SA and solutions B) and C) were used as water phase, stable W/O emulsion with water droplets which repel each other was made.
Comparing solutions B) and C), solution B) is more similar to solution A) in the concentration of particles. So we decided to use SDS as surfactant and water with 10mM glucose as water phase in the main experiment.
As continuous oil phase, a solution of liquid paraffin and squalene was used. The volume ratio of liquid paraffin to squalene was 1:1 and was prepared 5ml in total. Using high viscosity liquid paraffin stabilizes the droplets and mixing it with squalene that has relatively high viscosity makes the experiment proceed smoothly. Then 3.5mg (0.1 wt%) of SDS and 98.22mg (3wt%) of span80, which is a surfactant, were added. Because SDS is solid and it was difficult to dissolve it completely, the solution was warmed to 90 degrees Celsius for 30 minutes. The presence of SDS enhanced the dispensability of water droplets in oil (this solution was named solution A).
As water phase, two solutions in different conditions were prepared. For the first solution, 9mg of glucose was dissolved in pure water. In addition, EDTA and fluo3 were added and adjusted so that the concentration of each was 0.2 nM and 4.0μM (this was named solution B1). The reason why we used EDTA was clarifying that no calcium ions existed in solution B1. For the second solution, 27μl of 1.5M glucose solution mixed with 400μl of the 10wt% FMC (Fluoresbrite Carboxylate Microspheres) solution, which was washed with sodium hydroxide was prepared (this was named solution B2).
When preparing the water phase, FMS had to be calcified. Fluorescence beads in the FMC solution have carboxyl group and were provided as sodium compound. The compound was then immersed into dilute hydrochloric acid to separate carboxyl group and sodium. Consequently, it was reacted with calcium hydroxide neutralized with strong basic to generate fluorescence beads with calcified carboxyl group. The generated compound was then rinsed several times to wash off calcium ions that were not bonded with carboxyl group.
2. Saturate continuous oil phase with water
Solution A was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 (solution A:water=5.0ml:0.56ml) for 30 minutes. Solution A was saturated with water in advance in order to remove soluble impurities. (This avoids solution B1 and B2 from seeping into the continuous oil phase and affecting the fluorescence intensity observation at the end.) Then, the supernatant continuous oil phase was removed and distributed 1ml each into two micro-tubes, followed by centrifugation (5000rpm for 8min with a centrifugal separator). The supernatant solution was removed and used as continuous phase (the resulting solution was also named solution A).
3. Formation of water in oil emulsion(=W/O emulsion) by SPG pumping connector
Solutions A and B1 were each inserted into two syringes at the volume ratio of 4:1 (=600 μl:150μl in our experiment) and connected using the SPG pumping connector. The syringe of solution A was pushed towards solution B1 in order to coat the SPG porous surface of the SPQ pumping connector with solution A. In this way, solution B1 was forced through porous medium into solution A and solution B1 was dispersed. Through the process, the size of water droplets became almost the same as that of tunnels in the SPG pumping connector, which was about 2μm. Thus W/O emulsion was formed (named solution C1).
These figures are reffered from [4].
1. The continuous oil phase and the water phase were prepared and SPG pumping connecter was filled with the former in advance.
2. The syringe (piston) of the water phase was connected to the SPG pumping connecter.
3. The continuous oil phase was injected into the water phase.
4/5. The water phase was injected into the other side.
6. 1~5 was repeated until the size of the droplets was roughly 20 μl.
Reffered and translated from [4].
The same procedure was also conducted on solutions A and B2. (The resulting W/O emulsion was named solution C2). At this point, there should be two types of W/O emulsion (at the volume ratio of solution A:solution Bi = 4:1).
* i=1,2
4. Transfer of Calcium Ion using Ionomycin
Firstly, solutions C1 and C2 were mixed at the volume ratio of 1:1. Then two samples, one mixed with 0.1wt% ionomycin and the other with 0.02wt%, were prepared and extracted 100μl each (the solutions were named D1 and D2). Two additional samples made by mixing C1 & wt0.1 ionomycin and C1 & wt0.02% ionomycin were prepared as a contrast experiment (the solutions were named D3 and D4)
5. Observation
Solutions D1 ~ D4 were observed one hour and one day after preparation. Before observation, solution D was diluted 100 folds by solution A so that the final ratio of water phase:continuous oil phase was 1:400.
Preliminary Experiments
To conduct more efficient search and observation of W/O emulsion, we conducted some preliminary experiments before the main experiment.
These experiments were conducted with reference to the experiment by Sugiura and others (Sugiura et al. 2007).
Materials
Liquid paraffin
squalene
span80 (sorbitan monooleate)
SA (stearylamine)
calcein
Tris-HCl buffer
water
Experiments
1. Testing the practicality of the SPG pumping connector
The SPG (Shirasu Porous Glass) pumping connector is a device to make monodispersed W/O emulsion, in which droplets are uniform in size. Two kinds of SPG pumping connectors were prepared. The diameter of tunnels in one SPG pumping connector was 10μm and that of the other was 20μm. Although it is easier to observe bigger water droplets, it is more difficult for them to remain stable in general. To decide which SPG pumping connector to use in the main experiment, the stability of differently sized droplets was observed.
i. Preparation of solutions
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, Tris-HCl buffer (50mM,pH8) containing 0.4mM calcein was used.
ii. Saturate continuous oil phase with water
The continuous oil phase was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 for 30 minutes.
iii. Formation of W/O emulsion by SPG pumping connector
Continuous oil phase and water phase were each inserted into two syringes at the volume ratio of 10:1 and connected using the SPG pumping connector and were pumped until the size of the droplets became almost the same.
iv. Observation
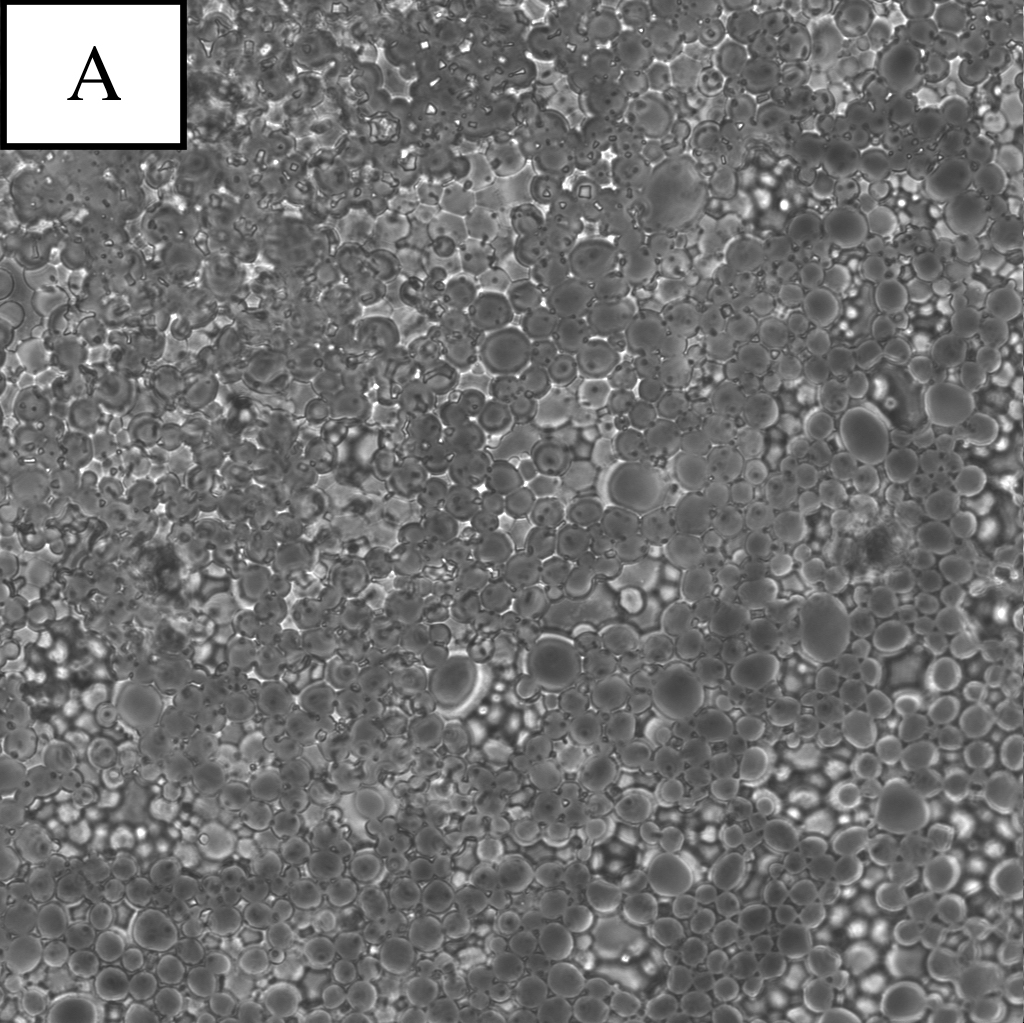
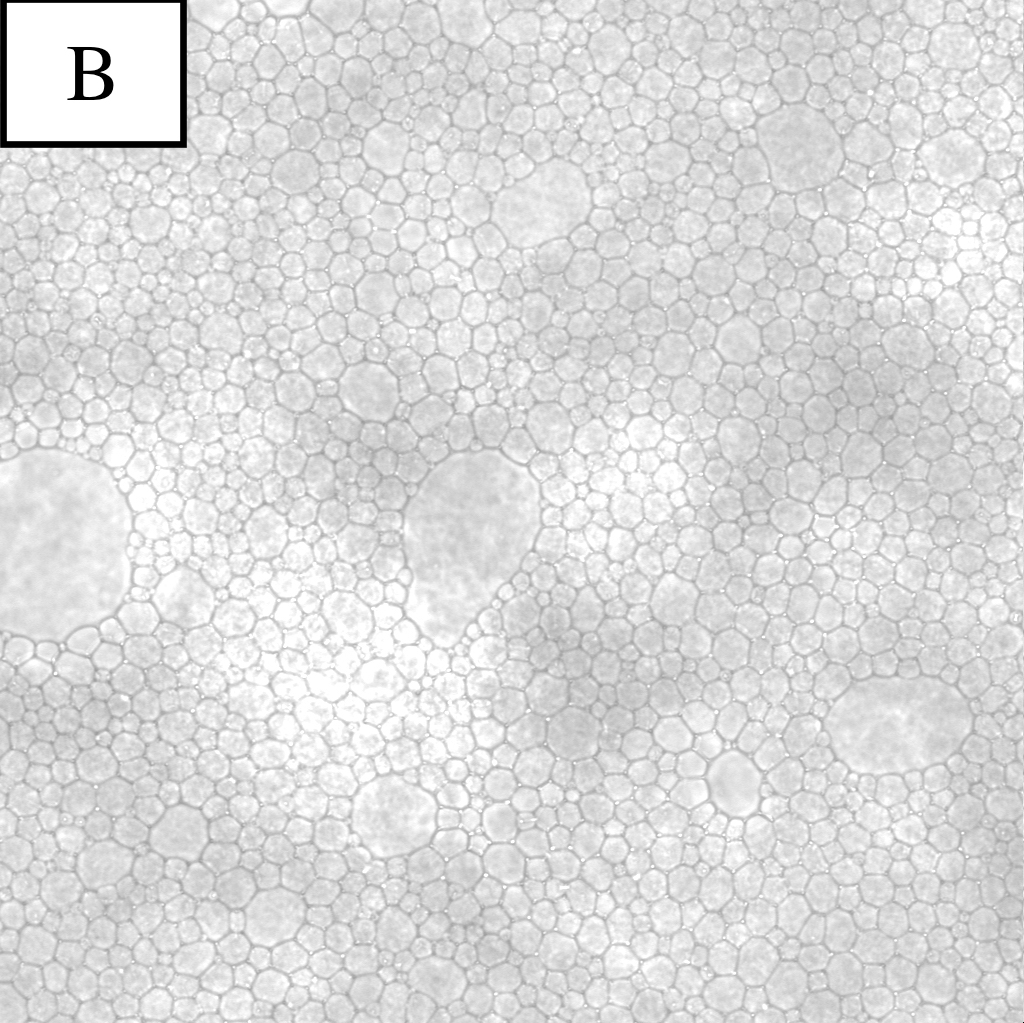
Figure 1. Microscope photographs of droplets.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
From the results of the observation of W/O emulsion, the following conclusions were obtained.
・Droplets produced by the connector with 20μm tunnels were stable enough to exist.
・By procedure 3, the diameter of droplets became certainly uniform.
・With more pumping, the proportion of smaller droplets became high.
Considering these results, we decided to use the SPG pumping connector with 20μm tunnels and to pump 5 times in the main experiment.
From these pictures, it was found that the density of droplets was too high and that it was important to search for proper density of droplets for observation.
2. Searching for proper density of droplets for observation
As the ratio of water phase to continuous phase changes, the density of droplets changes and the difficulty of observation also changes. For more efficient observation, four conditions of dilution were tested.
In this experiment, materials and procedures were almost the same as preliminary experiment 1. Only the degree of dilution before observation was different. After pumping, W/O emulsion was diluted with continuous phase to the degree that the ratio of water phase to continuous phase was 30:1, 100:1 and 1000:1.
Figure 2.
Microscope photographs of droplets at the different dilution rates.
Panel C is a picture taken in the bright field.
continuous oil phase:water phase=30:1
Panel D is a picture taken in the blue excitation light*.
continuous oil phase:water phase=100:1
Panel E is a picture taken in the blue excitation light*.
continuous oil phase:water phase=1000:1
*The wavelength of blue excitation light is close to the Maximal absorption wavelength of calcein. So in blue excitation light, calcein looks shining.
By observing W/O emulsion in these conditions, it was understood that the condition (water phase:continuous oil phase = 1:100~1000) was proper for observation.
3. Investigating the effect of Tris-HCl buffer to the stability of W/O emulsion
Tris-HCl buffer was used as water phase in the experiment by Sugiura. On the other hand, in our main experiment, Tris-HCl buffer was not proper because producing the difference of pH was important. To confirm whether W/O emulsion remains stable without buffer, and if not, to reconsider the conditions of the main experiment, we took place the following experiment.
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, the following solutions were used.
A) Tris-HCl buffer (pH9, 50mM)
B) Water with glucose (10mM) (No electrically charged particles exist. The concentration of particles in the solution was the same as that of 50mM Tris-HCl buffer)
C) Water
In solution A), stable W/O emulsion was observed. In other words, droplets repel each other and did not make clusters. In solutions B) and C), droplets were stuck together and many clusters were made. Thus, it became clear that in order for droplets to repel each other the existence of buffer was essential.
In this system, SA, which is a kind of amine, is the cause of repulsion between droplets and that of stability of W/O emulsion because amine is bound with a proton (H+ ion) ion in the solution and gets positive charge. It forms a part of the membrane of water droplets. Then, droplets get positive charge and repel each other.
From the above consideration, it became clear that in order for SA to work, providing SA with protons in the solution was essential. However, Tris-HCl buffer is not proper for the main experiment. Therefore, another kind of surfactant which causes repulsion between water droplets without ion interchange was needed.
Then, it occurred to us to use SDS instead of SA. SDS is surfactant made from strong acid and strong base, so SDS easily ionizes in water. Thus, it was expected to cause repulsion between water droplets in continuous oil phase.
When SDS was used as continuous oil phase in place of SA and solutions B) and C) were used as water phase, stable W/O emulsion with water droplets which repel each other was made.
Comparing solutions B) and C), solution B) is more similar to solution A) in the concentration of particles. So we decided to use SDS as surfactant and water with 10mM glucose as water phase in the main experiment.
Solution A was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 (solution A:water=5.0ml:0.56ml) for 30 minutes. Solution A was saturated with water in advance in order to remove soluble impurities. (This avoids solution B1 and B2 from seeping into the continuous oil phase and affecting the fluorescence intensity observation at the end.) Then, the supernatant continuous oil phase was removed and distributed 1ml each into two micro-tubes, followed by centrifugation (5000rpm for 8min with a centrifugal separator). The supernatant solution was removed and used as continuous phase (the resulting solution was also named solution A).
3. Formation of water in oil emulsion(=W/O emulsion) by SPG pumping connector
Solutions A and B1 were each inserted into two syringes at the volume ratio of 4:1 (=600 μl:150μl in our experiment) and connected using the SPG pumping connector. The syringe of solution A was pushed towards solution B1 in order to coat the SPG porous surface of the SPQ pumping connector with solution A. In this way, solution B1 was forced through porous medium into solution A and solution B1 was dispersed. Through the process, the size of water droplets became almost the same as that of tunnels in the SPG pumping connector, which was about 2μm. Thus W/O emulsion was formed (named solution C1).
These figures are reffered from [4].
1. The continuous oil phase and the water phase were prepared and SPG pumping connecter was filled with the former in advance.
2. The syringe (piston) of the water phase was connected to the SPG pumping connecter.
3. The continuous oil phase was injected into the water phase.
4/5. The water phase was injected into the other side.
6. 1~5 was repeated until the size of the droplets was roughly 20 μl.
Reffered and translated from [4].
The same procedure was also conducted on solutions A and B2. (The resulting W/O emulsion was named solution C2). At this point, there should be two types of W/O emulsion (at the volume ratio of solution A:solution Bi = 4:1).
* i=1,2
4. Transfer of Calcium Ion using Ionomycin
Firstly, solutions C1 and C2 were mixed at the volume ratio of 1:1. Then two samples, one mixed with 0.1wt% ionomycin and the other with 0.02wt%, were prepared and extracted 100μl each (the solutions were named D1 and D2). Two additional samples made by mixing C1 & wt0.1 ionomycin and C1 & wt0.02% ionomycin were prepared as a contrast experiment (the solutions were named D3 and D4)
5. Observation
Solutions D1 ~ D4 were observed one hour and one day after preparation. Before observation, solution D was diluted 100 folds by solution A so that the final ratio of water phase:continuous oil phase was 1:400.
Preliminary Experiments
To conduct more efficient search and observation of W/O emulsion, we conducted some preliminary experiments before the main experiment.
These experiments were conducted with reference to the experiment by Sugiura and others (Sugiura et al. 2007).
Materials
Liquid paraffin
squalene
span80 (sorbitan monooleate)
SA (stearylamine)
calcein
Tris-HCl buffer
water
Experiments
1. Testing the practicality of the SPG pumping connector
The SPG (Shirasu Porous Glass) pumping connector is a device to make monodispersed W/O emulsion, in which droplets are uniform in size. Two kinds of SPG pumping connectors were prepared. The diameter of tunnels in one SPG pumping connector was 10μm and that of the other was 20μm. Although it is easier to observe bigger water droplets, it is more difficult for them to remain stable in general. To decide which SPG pumping connector to use in the main experiment, the stability of differently sized droplets was observed.
i. Preparation of solutions
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, Tris-HCl buffer (50mM,pH8) containing 0.4mM calcein was used.
ii. Saturate continuous oil phase with water
The continuous oil phase was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 for 30 minutes.
iii. Formation of W/O emulsion by SPG pumping connector
Continuous oil phase and water phase were each inserted into two syringes at the volume ratio of 10:1 and connected using the SPG pumping connector and were pumped until the size of the droplets became almost the same.
iv. Observation
Figure 1. Microscope photographs of droplets.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
From the results of the observation of W/O emulsion, the following conclusions were obtained.
・Droplets produced by the connector with 20μm tunnels were stable enough to exist.
・By procedure 3, the diameter of droplets became certainly uniform.
・With more pumping, the proportion of smaller droplets became high.
Considering these results, we decided to use the SPG pumping connector with 20μm tunnels and to pump 5 times in the main experiment.
From these pictures, it was found that the density of droplets was too high and that it was important to search for proper density of droplets for observation.
2. Searching for proper density of droplets for observation
As the ratio of water phase to continuous phase changes, the density of droplets changes and the difficulty of observation also changes. For more efficient observation, four conditions of dilution were tested.
In this experiment, materials and procedures were almost the same as preliminary experiment 1. Only the degree of dilution before observation was different. After pumping, W/O emulsion was diluted with continuous phase to the degree that the ratio of water phase to continuous phase was 30:1, 100:1 and 1000:1.
Figure 2.
Microscope photographs of droplets at the different dilution rates.
Panel C is a picture taken in the bright field.
continuous oil phase:water phase=30:1
Panel D is a picture taken in the blue excitation light*.
continuous oil phase:water phase=100:1
Panel E is a picture taken in the blue excitation light*.
continuous oil phase:water phase=1000:1
*The wavelength of blue excitation light is close to the Maximal absorption wavelength of calcein. So in blue excitation light, calcein looks shining.
By observing W/O emulsion in these conditions, it was understood that the condition (water phase:continuous oil phase = 1:100~1000) was proper for observation.
3. Investigating the effect of Tris-HCl buffer to the stability of W/O emulsion
Tris-HCl buffer was used as water phase in the experiment by Sugiura. On the other hand, in our main experiment, Tris-HCl buffer was not proper because producing the difference of pH was important. To confirm whether W/O emulsion remains stable without buffer, and if not, to reconsider the conditions of the main experiment, we took place the following experiment.
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, the following solutions were used.
A) Tris-HCl buffer (pH9, 50mM)
B) Water with glucose (10mM) (No electrically charged particles exist. The concentration of particles in the solution was the same as that of 50mM Tris-HCl buffer)
C) Water
In solution A), stable W/O emulsion was observed. In other words, droplets repel each other and did not make clusters. In solutions B) and C), droplets were stuck together and many clusters were made. Thus, it became clear that in order for droplets to repel each other the existence of buffer was essential.
In this system, SA, which is a kind of amine, is the cause of repulsion between droplets and that of stability of W/O emulsion because amine is bound with a proton (H+ ion) ion in the solution and gets positive charge. It forms a part of the membrane of water droplets. Then, droplets get positive charge and repel each other.
From the above consideration, it became clear that in order for SA to work, providing SA with protons in the solution was essential. However, Tris-HCl buffer is not proper for the main experiment. Therefore, another kind of surfactant which causes repulsion between water droplets without ion interchange was needed.
Then, it occurred to us to use SDS instead of SA. SDS is surfactant made from strong acid and strong base, so SDS easily ionizes in water. Thus, it was expected to cause repulsion between water droplets in continuous oil phase.
When SDS was used as continuous oil phase in place of SA and solutions B) and C) were used as water phase, stable W/O emulsion with water droplets which repel each other was made.
Comparing solutions B) and C), solution B) is more similar to solution A) in the concentration of particles. So we decided to use SDS as surfactant and water with 10mM glucose as water phase in the main experiment.
Solutions A and B1 were each inserted into two syringes at the volume ratio of 4:1 (=600 μl:150μl in our experiment) and connected using the SPG pumping connector. The syringe of solution A was pushed towards solution B1 in order to coat the SPG porous surface of the SPQ pumping connector with solution A. In this way, solution B1 was forced through porous medium into solution A and solution B1 was dispersed. Through the process, the size of water droplets became almost the same as that of tunnels in the SPG pumping connector, which was about 2μm. Thus W/O emulsion was formed (named solution C1).
These figures are reffered from [4].
1. The continuous oil phase and the water phase were prepared and SPG pumping connecter was filled with the former in advance.
2. The syringe (piston) of the water phase was connected to the SPG pumping connecter.
3. The continuous oil phase was injected into the water phase.
4/5. The water phase was injected into the other side.
6. 1~5 was repeated until the size of the droplets was roughly 20 μl.
Reffered and translated from [4].
The same procedure was also conducted on solutions A and B2. (The resulting W/O emulsion was named solution C2). At this point, there should be two types of W/O emulsion (at the volume ratio of solution A:solution Bi = 4:1).
* i=1,2
4. Transfer of Calcium Ion using Ionomycin
Firstly, solutions C1 and C2 were mixed at the volume ratio of 1:1. Then two samples, one mixed with 0.1wt% ionomycin and the other with 0.02wt%, were prepared and extracted 100μl each (the solutions were named D1 and D2). Two additional samples made by mixing C1 & wt0.1 ionomycin and C1 & wt0.02% ionomycin were prepared as a contrast experiment (the solutions were named D3 and D4)
5. Observation
Solutions D1 ~ D4 were observed one hour and one day after preparation. Before observation, solution D was diluted 100 folds by solution A so that the final ratio of water phase:continuous oil phase was 1:400.
Preliminary Experiments
To conduct more efficient search and observation of W/O emulsion, we conducted some preliminary experiments before the main experiment.
These experiments were conducted with reference to the experiment by Sugiura and others (Sugiura et al. 2007).
Materials
Liquid paraffin
squalene
span80 (sorbitan monooleate)
SA (stearylamine)
calcein
Tris-HCl buffer
water
Experiments
1. Testing the practicality of the SPG pumping connector
The SPG (Shirasu Porous Glass) pumping connector is a device to make monodispersed W/O emulsion, in which droplets are uniform in size. Two kinds of SPG pumping connectors were prepared. The diameter of tunnels in one SPG pumping connector was 10μm and that of the other was 20μm. Although it is easier to observe bigger water droplets, it is more difficult for them to remain stable in general. To decide which SPG pumping connector to use in the main experiment, the stability of differently sized droplets was observed.
i. Preparation of solutions
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, Tris-HCl buffer (50mM,pH8) containing 0.4mM calcein was used.
ii. Saturate continuous oil phase with water
The continuous oil phase was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 for 30 minutes.
iii. Formation of W/O emulsion by SPG pumping connector
Continuous oil phase and water phase were each inserted into two syringes at the volume ratio of 10:1 and connected using the SPG pumping connector and were pumped until the size of the droplets became almost the same.
iv. Observation
Figure 1. Microscope photographs of droplets.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
From the results of the observation of W/O emulsion, the following conclusions were obtained.
・Droplets produced by the connector with 20μm tunnels were stable enough to exist.
・By procedure 3, the diameter of droplets became certainly uniform.
・With more pumping, the proportion of smaller droplets became high.
Considering these results, we decided to use the SPG pumping connector with 20μm tunnels and to pump 5 times in the main experiment.
From these pictures, it was found that the density of droplets was too high and that it was important to search for proper density of droplets for observation.
2. Searching for proper density of droplets for observation
As the ratio of water phase to continuous phase changes, the density of droplets changes and the difficulty of observation also changes. For more efficient observation, four conditions of dilution were tested.
In this experiment, materials and procedures were almost the same as preliminary experiment 1. Only the degree of dilution before observation was different. After pumping, W/O emulsion was diluted with continuous phase to the degree that the ratio of water phase to continuous phase was 30:1, 100:1 and 1000:1.
Figure 2.
Microscope photographs of droplets at the different dilution rates.
Panel C is a picture taken in the bright field.
continuous oil phase:water phase=30:1
Panel D is a picture taken in the blue excitation light*.
continuous oil phase:water phase=100:1
Panel E is a picture taken in the blue excitation light*.
continuous oil phase:water phase=1000:1
*The wavelength of blue excitation light is close to the Maximal absorption wavelength of calcein. So in blue excitation light, calcein looks shining.
By observing W/O emulsion in these conditions, it was understood that the condition (water phase:continuous oil phase = 1:100~1000) was proper for observation.
3. Investigating the effect of Tris-HCl buffer to the stability of W/O emulsion
Tris-HCl buffer was used as water phase in the experiment by Sugiura. On the other hand, in our main experiment, Tris-HCl buffer was not proper because producing the difference of pH was important. To confirm whether W/O emulsion remains stable without buffer, and if not, to reconsider the conditions of the main experiment, we took place the following experiment.
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, the following solutions were used.
A) Tris-HCl buffer (pH9, 50mM)
B) Water with glucose (10mM) (No electrically charged particles exist. The concentration of particles in the solution was the same as that of 50mM Tris-HCl buffer)
C) Water
In solution A), stable W/O emulsion was observed. In other words, droplets repel each other and did not make clusters. In solutions B) and C), droplets were stuck together and many clusters were made. Thus, it became clear that in order for droplets to repel each other the existence of buffer was essential.
In this system, SA, which is a kind of amine, is the cause of repulsion between droplets and that of stability of W/O emulsion because amine is bound with a proton (H+ ion) ion in the solution and gets positive charge. It forms a part of the membrane of water droplets. Then, droplets get positive charge and repel each other.
From the above consideration, it became clear that in order for SA to work, providing SA with protons in the solution was essential. However, Tris-HCl buffer is not proper for the main experiment. Therefore, another kind of surfactant which causes repulsion between water droplets without ion interchange was needed.
Then, it occurred to us to use SDS instead of SA. SDS is surfactant made from strong acid and strong base, so SDS easily ionizes in water. Thus, it was expected to cause repulsion between water droplets in continuous oil phase.
When SDS was used as continuous oil phase in place of SA and solutions B) and C) were used as water phase, stable W/O emulsion with water droplets which repel each other was made.
Comparing solutions B) and C), solution B) is more similar to solution A) in the concentration of particles. So we decided to use SDS as surfactant and water with 10mM glucose as water phase in the main experiment.
These figures are reffered from [4].
1. The continuous oil phase and the water phase were prepared and SPG pumping connecter was filled with the former in advance.
2. The syringe (piston) of the water phase was connected to the SPG pumping connecter.
3. The continuous oil phase was injected into the water phase.
4/5. The water phase was injected into the other side.
6. 1~5 was repeated until the size of the droplets was roughly 20 μl.
Reffered and translated from [4].
The same procedure was also conducted on solutions A and B2. (The resulting W/O emulsion was named solution C2). At this point, there should be two types of W/O emulsion (at the volume ratio of solution A:solution Bi = 4:1).
* i=1,2
4. Transfer of Calcium Ion using Ionomycin
Firstly, solutions C1 and C2 were mixed at the volume ratio of 1:1. Then two samples, one mixed with 0.1wt% ionomycin and the other with 0.02wt%, were prepared and extracted 100μl each (the solutions were named D1 and D2). Two additional samples made by mixing C1 & wt0.1 ionomycin and C1 & wt0.02% ionomycin were prepared as a contrast experiment (the solutions were named D3 and D4)
5. Observation
Solutions D1 ~ D4 were observed one hour and one day after preparation. Before observation, solution D was diluted 100 folds by solution A so that the final ratio of water phase:continuous oil phase was 1:400.
Firstly, solutions C1 and C2 were mixed at the volume ratio of 1:1. Then two samples, one mixed with 0.1wt% ionomycin and the other with 0.02wt%, were prepared and extracted 100μl each (the solutions were named D1 and D2). Two additional samples made by mixing C1 & wt0.1 ionomycin and C1 & wt0.02% ionomycin were prepared as a contrast experiment (the solutions were named D3 and D4)
5. Observation
Solutions D1 ~ D4 were observed one hour and one day after preparation. Before observation, solution D was diluted 100 folds by solution A so that the final ratio of water phase:continuous oil phase was 1:400.
Solutions D1 ~ D4 were observed one hour and one day after preparation. Before observation, solution D was diluted 100 folds by solution A so that the final ratio of water phase:continuous oil phase was 1:400.
Preliminary Experiments
To conduct more efficient search and observation of W/O emulsion, we conducted some preliminary experiments before the main experiment. These experiments were conducted with reference to the experiment by Sugiura and others (Sugiura et al. 2007).
Materials
Liquid paraffin
squalene
span80 (sorbitan monooleate)
SA (stearylamine)
calcein
Tris-HCl buffer
water
Experiments
1. Testing the practicality of the SPG pumping connector
The SPG (Shirasu Porous Glass) pumping connector is a device to make monodispersed W/O emulsion, in which droplets are uniform in size. Two kinds of SPG pumping connectors were prepared. The diameter of tunnels in one SPG pumping connector was 10μm and that of the other was 20μm. Although it is easier to observe bigger water droplets, it is more difficult for them to remain stable in general. To decide which SPG pumping connector to use in the main experiment, the stability of differently sized droplets was observed.
i. Preparation of solutions
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, Tris-HCl buffer (50mM,pH8) containing 0.4mM calcein was used.
ii. Saturate continuous oil phase with water
The continuous oil phase was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 for 30 minutes.
iii. Formation of W/O emulsion by SPG pumping connector
Continuous oil phase and water phase were each inserted into two syringes at the volume ratio of 10:1 and connected using the SPG pumping connector and were pumped until the size of the droplets became almost the same.
iv. Observation
Figure 1. Microscope photographs of droplets.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
From the results of the observation of W/O emulsion, the following conclusions were obtained.
・Droplets produced by the connector with 20μm tunnels were stable enough to exist.
・By procedure 3, the diameter of droplets became certainly uniform.
・With more pumping, the proportion of smaller droplets became high.
Considering these results, we decided to use the SPG pumping connector with 20μm tunnels and to pump 5 times in the main experiment.
From these pictures, it was found that the density of droplets was too high and that it was important to search for proper density of droplets for observation.
2. Searching for proper density of droplets for observation
As the ratio of water phase to continuous phase changes, the density of droplets changes and the difficulty of observation also changes. For more efficient observation, four conditions of dilution were tested.
In this experiment, materials and procedures were almost the same as preliminary experiment 1. Only the degree of dilution before observation was different. After pumping, W/O emulsion was diluted with continuous phase to the degree that the ratio of water phase to continuous phase was 30:1, 100:1 and 1000:1.
Figure 2.
Microscope photographs of droplets at the different dilution rates.
Panel C is a picture taken in the bright field.
continuous oil phase:water phase=30:1
Panel D is a picture taken in the blue excitation light*.
continuous oil phase:water phase=100:1
Panel E is a picture taken in the blue excitation light*.
continuous oil phase:water phase=1000:1
*The wavelength of blue excitation light is close to the Maximal absorption wavelength of calcein. So in blue excitation light, calcein looks shining.
By observing W/O emulsion in these conditions, it was understood that the condition (water phase:continuous oil phase = 1:100~1000) was proper for observation.
3. Investigating the effect of Tris-HCl buffer to the stability of W/O emulsion
Tris-HCl buffer was used as water phase in the experiment by Sugiura. On the other hand, in our main experiment, Tris-HCl buffer was not proper because producing the difference of pH was important. To confirm whether W/O emulsion remains stable without buffer, and if not, to reconsider the conditions of the main experiment, we took place the following experiment.
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, the following solutions were used.
A) Tris-HCl buffer (pH9, 50mM)
B) Water with glucose (10mM) (No electrically charged particles exist. The concentration of particles in the solution was the same as that of 50mM Tris-HCl buffer)
C) Water
In solution A), stable W/O emulsion was observed. In other words, droplets repel each other and did not make clusters. In solutions B) and C), droplets were stuck together and many clusters were made. Thus, it became clear that in order for droplets to repel each other the existence of buffer was essential.
In this system, SA, which is a kind of amine, is the cause of repulsion between droplets and that of stability of W/O emulsion because amine is bound with a proton (H+ ion) ion in the solution and gets positive charge. It forms a part of the membrane of water droplets. Then, droplets get positive charge and repel each other.
From the above consideration, it became clear that in order for SA to work, providing SA with protons in the solution was essential. However, Tris-HCl buffer is not proper for the main experiment. Therefore, another kind of surfactant which causes repulsion between water droplets without ion interchange was needed.
Then, it occurred to us to use SDS instead of SA. SDS is surfactant made from strong acid and strong base, so SDS easily ionizes in water. Thus, it was expected to cause repulsion between water droplets in continuous oil phase.
When SDS was used as continuous oil phase in place of SA and solutions B) and C) were used as water phase, stable W/O emulsion with water droplets which repel each other was made.
Comparing solutions B) and C), solution B) is more similar to solution A) in the concentration of particles. So we decided to use SDS as surfactant and water with 10mM glucose as water phase in the main experiment.
1. Testing the practicality of the SPG pumping connector
The SPG (Shirasu Porous Glass) pumping connector is a device to make monodispersed W/O emulsion, in which droplets are uniform in size. Two kinds of SPG pumping connectors were prepared. The diameter of tunnels in one SPG pumping connector was 10μm and that of the other was 20μm. Although it is easier to observe bigger water droplets, it is more difficult for them to remain stable in general. To decide which SPG pumping connector to use in the main experiment, the stability of differently sized droplets was observed.
i. Preparation of solutions
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, Tris-HCl buffer (50mM,pH8) containing 0.4mM calcein was used.
ii. Saturate continuous oil phase with water
The continuous oil phase was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 for 30 minutes.
iii. Formation of W/O emulsion by SPG pumping connector
Continuous oil phase and water phase were each inserted into two syringes at the volume ratio of 10:1 and connected using the SPG pumping connector and were pumped until the size of the droplets became almost the same.
iv. Observation
Figure 1. Microscope photographs of droplets.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
From the results of the observation of W/O emulsion, the following conclusions were obtained.
・Droplets produced by the connector with 20μm tunnels were stable enough to exist.
・By procedure 3, the diameter of droplets became certainly uniform.
・With more pumping, the proportion of smaller droplets became high.
Considering these results, we decided to use the SPG pumping connector with 20μm tunnels and to pump 5 times in the main experiment.
From these pictures, it was found that the density of droplets was too high and that it was important to search for proper density of droplets for observation.
i. Preparation of solutions
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, Tris-HCl buffer (50mM,pH8) containing 0.4mM calcein was used.
ii. Saturate continuous oil phase with water
The continuous oil phase was saturated with water by letting solution A be in contact with water at a volume ratio of 9:1 for 30 minutes.
iii. Formation of W/O emulsion by SPG pumping connector
Continuous oil phase and water phase were each inserted into two syringes at the volume ratio of 10:1 and connected using the SPG pumping connector and were pumped until the size of the droplets became almost the same.
iv. Observation
Figure 1. Microscope photographs of droplets.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
Figure 1. Microscope photographs of droplets.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
Panel A is a picture of droplets made by the pumping connector with 10μm tunnels.
Panel B is a picture of droplets made by the pumping connector with 20μm tunnels.
2. Searching for proper density of droplets for observation
As the ratio of water phase to continuous phase changes, the density of droplets changes and the difficulty of observation also changes. For more efficient observation, four conditions of dilution were tested.
In this experiment, materials and procedures were almost the same as preliminary experiment 1. Only the degree of dilution before observation was different. After pumping, W/O emulsion was diluted with continuous phase to the degree that the ratio of water phase to continuous phase was 30:1, 100:1 and 1000:1.
Figure 2.
Microscope photographs of droplets at the different dilution rates.
Panel C is a picture taken in the bright field.
continuous oil phase:water phase=30:1
Panel D is a picture taken in the blue excitation light*.
continuous oil phase:water phase=100:1
Panel E is a picture taken in the blue excitation light*.
continuous oil phase:water phase=1000:1
*The wavelength of blue excitation light is close to the Maximal absorption wavelength of calcein. So in blue excitation light, calcein looks shining.
By observing W/O emulsion in these conditions, it was understood that the condition (water phase:continuous oil phase = 1:100~1000) was proper for observation.
3. Investigating the effect of Tris-HCl buffer to the stability of W/O emulsion
Tris-HCl buffer was used as water phase in the experiment by Sugiura. On the other hand, in our main experiment, Tris-HCl buffer was not proper because producing the difference of pH was important. To confirm whether W/O emulsion remains stable without buffer, and if not, to reconsider the conditions of the main experiment, we took place the following experiment.
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, the following solutions were used.
A) Tris-HCl buffer (pH9, 50mM)
B) Water with glucose (10mM) (No electrically charged particles exist. The concentration of particles in the solution was the same as that of 50mM Tris-HCl buffer)
C) Water
In solution A), stable W/O emulsion was observed. In other words, droplets repel each other and did not make clusters. In solutions B) and C), droplets were stuck together and many clusters were made. Thus, it became clear that in order for droplets to repel each other the existence of buffer was essential.
In this system, SA, which is a kind of amine, is the cause of repulsion between droplets and that of stability of W/O emulsion because amine is bound with a proton (H+ ion) ion in the solution and gets positive charge. It forms a part of the membrane of water droplets. Then, droplets get positive charge and repel each other.
From the above consideration, it became clear that in order for SA to work, providing SA with protons in the solution was essential. However, Tris-HCl buffer is not proper for the main experiment. Therefore, another kind of surfactant which causes repulsion between water droplets without ion interchange was needed.
Then, it occurred to us to use SDS instead of SA. SDS is surfactant made from strong acid and strong base, so SDS easily ionizes in water. Thus, it was expected to cause repulsion between water droplets in continuous oil phase.
When SDS was used as continuous oil phase in place of SA and solutions B) and C) were used as water phase, stable W/O emulsion with water droplets which repel each other was made.
Comparing solutions B) and C), solution B) is more similar to solution A) in the concentration of particles. So we decided to use SDS as surfactant and water with 10mM glucose as water phase in the main experiment.
Figure 2.
Microscope photographs of droplets at the different dilution rates.
Panel C is a picture taken in the bright field.
continuous oil phase:water phase=30:1
Panel D is a picture taken in the blue excitation light*.
continuous oil phase:water phase=100:1
Panel E is a picture taken in the blue excitation light*.
continuous oil phase:water phase=1000:1
*The wavelength of blue excitation light is close to the Maximal absorption wavelength of calcein. So in blue excitation light, calcein looks shining.
By observing W/O emulsion in these conditions, it was understood that the condition (water phase:continuous oil phase = 1:100~1000) was proper for observation.
3. Investigating the effect of Tris-HCl buffer to the stability of W/O emulsion
Tris-HCl buffer was used as water phase in the experiment by Sugiura. On the other hand, in our main experiment, Tris-HCl buffer was not proper because producing the difference of pH was important. To confirm whether W/O emulsion remains stable without buffer, and if not, to reconsider the conditions of the main experiment, we took place the following experiment.
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, the following solutions were used.
A) Tris-HCl buffer (pH9, 50mM)
B) Water with glucose (10mM) (No electrically charged particles exist. The concentration of particles in the solution was the same as that of 50mM Tris-HCl buffer)
C) Water
In solution A), stable W/O emulsion was observed. In other words, droplets repel each other and did not make clusters. In solutions B) and C), droplets were stuck together and many clusters were made. Thus, it became clear that in order for droplets to repel each other the existence of buffer was essential.
In this system, SA, which is a kind of amine, is the cause of repulsion between droplets and that of stability of W/O emulsion because amine is bound with a proton (H+ ion) ion in the solution and gets positive charge. It forms a part of the membrane of water droplets. Then, droplets get positive charge and repel each other.
From the above consideration, it became clear that in order for SA to work, providing SA with protons in the solution was essential. However, Tris-HCl buffer is not proper for the main experiment. Therefore, another kind of surfactant which causes repulsion between water droplets without ion interchange was needed.
Then, it occurred to us to use SDS instead of SA. SDS is surfactant made from strong acid and strong base, so SDS easily ionizes in water. Thus, it was expected to cause repulsion between water droplets in continuous oil phase.
When SDS was used as continuous oil phase in place of SA and solutions B) and C) were used as water phase, stable W/O emulsion with water droplets which repel each other was made.
Comparing solutions B) and C), solution B) is more similar to solution A) in the concentration of particles. So we decided to use SDS as surfactant and water with 10mM glucose as water phase in the main experiment.
As continuous oil phase, the same volume of liquid paraffin and squalen were mixed and 3wt% span80 and 0.1wt% SA were added. As water phase, the following solutions were used.
A) Tris-HCl buffer (pH9, 50mM)
B) Water with glucose (10mM) (No electrically charged particles exist. The concentration of particles in the solution was the same as that of 50mM Tris-HCl buffer)
C) Water
In solution A), stable W/O emulsion was observed. In other words, droplets repel each other and did not make clusters. In solutions B) and C), droplets were stuck together and many clusters were made. Thus, it became clear that in order for droplets to repel each other the existence of buffer was essential.
In this system, SA, which is a kind of amine, is the cause of repulsion between droplets and that of stability of W/O emulsion because amine is bound with a proton (H+ ion) ion in the solution and gets positive charge. It forms a part of the membrane of water droplets. Then, droplets get positive charge and repel each other.
From the above consideration, it became clear that in order for SA to work, providing SA with protons in the solution was essential. However, Tris-HCl buffer is not proper for the main experiment. Therefore, another kind of surfactant which causes repulsion between water droplets without ion interchange was needed.
Then, it occurred to us to use SDS instead of SA. SDS is surfactant made from strong acid and strong base, so SDS easily ionizes in water. Thus, it was expected to cause repulsion between water droplets in continuous oil phase.
When SDS was used as continuous oil phase in place of SA and solutions B) and C) were used as water phase, stable W/O emulsion with water droplets which repel each other was made.
Comparing solutions B) and C), solution B) is more similar to solution A) in the concentration of particles. So we decided to use SDS as surfactant and water with 10mM glucose as water phase in the main experiment.