Micro-Bio-Battery
Abstract
In the natural world, any system in an unstable state shifts to the most stable state, i.e., from a state with high free energy to that with the lowest. This natural phenomenon proceeds so as to minimize the entire free energy, and the lowest energy state is called the equilibrium. Especially in the micro world, every molecule moves to form a more disordered state toward the equilibrium. This movement attributes to the law of entropy increase. In this project, we propose a new kind of device made of tiny water droplets enclosed by lipid layer in oil phase, which can extract the free energy produced in the process of entropy increase as a proton (H+ ion) concentration gradient in a pair of the droplets.
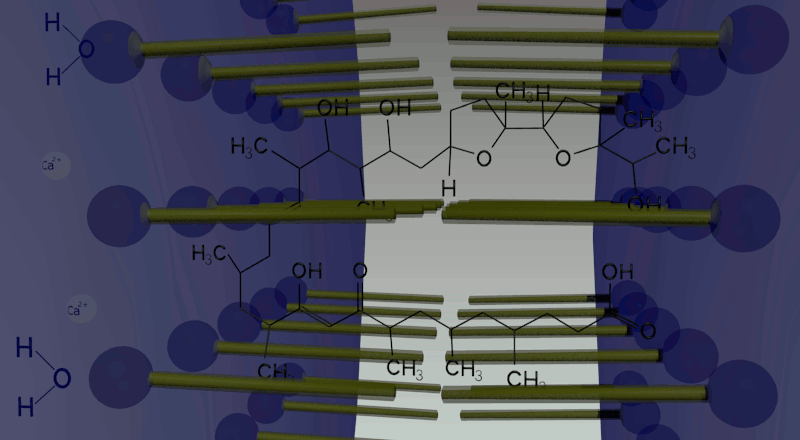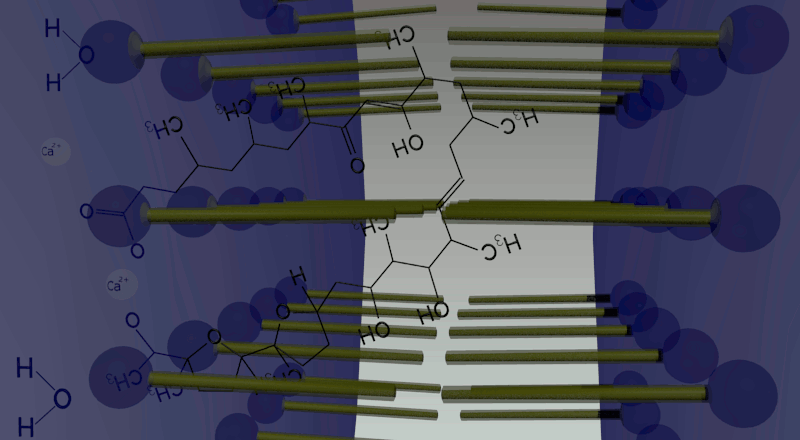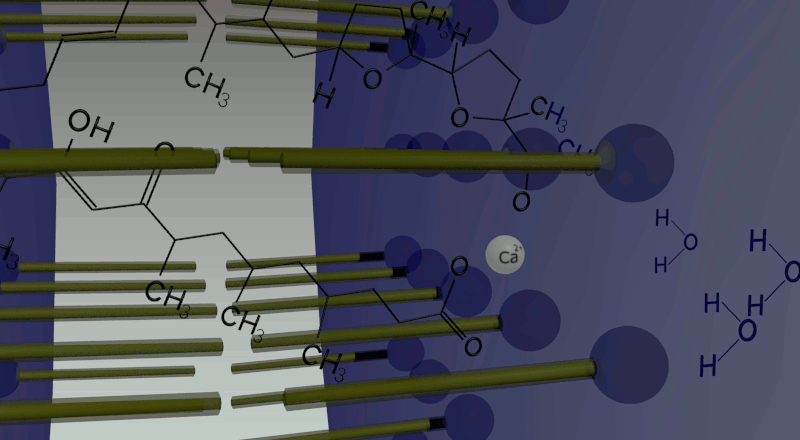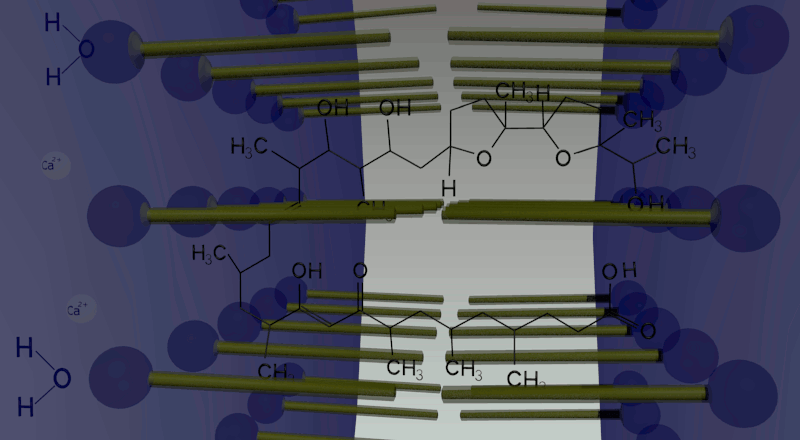
In oil phase, two water droplets are joined. Calcium ions (cations) and giant anionic fluorescent beads are put inside one of the two droplets, while calcium indicator inside the other with no ions. Ionomycin is put in the oil phase. Because of the law of entropy increase, in order to equate the ion concentration between the two droplets, Ca2+ ions in one droplet move to the other side by making complex with ionomycin. Arriving in the other droplet, Ca2+ ions combined with ionomycin are replaced by protons produced by dissociation of water molecules in the droplet. Then ionomycin flows into the oil phase. This movement is repeated again and again until the concentration of Ca2+ ions in both droplets becomes equal. In total, one droplet releases Ca2+ ions and gets H+ ions, while the other releases H+ ions and gets Ca2+ ions. Consequently, a proton concentration gradient is produced.
In the future, with the help of F0F1-ATPase, this device will produce ATP. All living things from bacteria to human beings use ATP (Adenosine Triphosphate) as energy source. So it can be said that this device works as a battery in our body in that it supplies ATP, i.e., the energy source of all biological activities.
Our first goal in this project is to make two kinds of droplets, one with calcium ions and anionic fluorescent beads, and the other with calcium indicator. The second goal is to observe movement of Ca2+ ions from the former kind of droplet to the latter owing to the law of entropy increase mentioned above. We successfully confirmed the movement by microscope photographs taken in the bright field and two different excitation lights.